State educational institution average comprehensive school № 325
Frunzensky district of St. Petersburg
ABSTRACT ON CHEMISTRY
"ALUMINIUM OXIDE"
The work was done by student 9 "A"
Ershova Maria
Scientific adviser:
Rogova E.V.
Scientific consultant:
Golovko N.V.
St. Petersburg
1. Introduction……………………………………………………….3
3. Precious stones and their formation……………………….6
Corundum..………………………………………………….……..6
Sapphires………………………………………………………..7
Rubies…………………………………………………….……8
4. Physical Properties……………………………………....…..9
5. Chemical properties ……………………..............................10
6. Application…………………….. ………………………..…..12
Production of aluminum…………………………………….…..12
Use of the physical properties of Al 2 O 3 ………………......15
7. Conclusion……………………………………………….…....17
8. Information sources…………………………..……..18
Introduction
Jewelry- a symbol of special status, they adorn the crowns of kings, as a visual reminder of the wealth and prosperity of the one who wears them, as well as those who see it.
Witch doctors and healers attribute to some precious stones magical power. Legends about the famous stones, about the happiness and troubles that they brought to their owners, captivate our imagination.
Gems- that's the treasure. Them unique properties appreciated at all times and around the world. They are valued to this day. Perhaps the attention of the one who first picked up the gem was attracted by its bright color or crystalline form, or perhaps the color of the rock in which it was enclosed.
The variety of colors of corundum enchants and attracts most people, especially ladies.
Pure corundum is colorless. However, corundum is used not only as a jewel, there are many more uses for this mineral. Or rather, what it consists of.
Objective: to analyze the properties of aluminum oxide and its application in industry and in everyday life.
Tasks :
1. Analyze the literature on the topic "Aluminum Oxide"
2. To study the historical aspect of the phenomenon of matter.
3. Explore the application of aluminum oxide.
4. Draw conclusions about the topic.
In terms of prevalence in earth's crust aluminum ranks first among metals and third among all elements (after oxygen and silicon), it accounts for about 8.8% of the mass of the earth's crust. There is twice as much aluminum as iron and 350 times as much as copper, zinc, chromium, tin and lead combined! Aluminum is included in a huge number of minerals, mainly aluminosilicates, and rocks. Aluminum compounds contain granites, basalts, clays, feldspars, etc. In total, more than 250 minerals are known that contain aluminum; most of them are aluminosilicates, from which the earth's crust is mainly formed.
Aluminum compounds have been known to man since ancient times. One of them were binders, which include alumina-potassium alum КAl(SO 4) 2 . They have been widely used. Used as a mordant and as a blood stopper. Impregnation of wood with a solution of potassium alum made it non-combustible. Known interesting historical fact. Archelaus, a commander from Rome, during the war with the Persians, ordered to smear the towers, which served as defensive structures, with alum. The Persians never succeeded in burning them.
During the weathering of aluminosilicates, clay, which is based on the mineral kaolinite Al 2 O 3 2SiO 2 2H 2 O. Chemical composition clay varies over a wide range, and the oxides that make up clays affect the process of obtaining the final properties of ceramics in different ways. Aluminum oxide (alumina - A1 2 0 3) with its increased amount in clay leads to an increase in the firing temperature and sintering interval. And products with a low alumina content have low strength. Iron impurities usually color the clay brown, but white clay is also found - kaolin, which is used to make porcelain and faience products.
Alumina, Al 2 O s - white crystalline substance, insoluble in water, t pl \u003d 2050 o C. Occurs in nature in the form of minerals - corundum(colorless), ruby (red), sapphire (blue). We will talk about corundum in the next chapter.
The most important mineral of aluminum is bauxite, Al 2 O 3 xH 2 O. The largest deposits of bauxite are in Australia, Brazil, Guinea and Jamaica; industrial production is also carried out in other countries. Bauxite (fr. bauxite) (by the name of the area Baux in the south of France) is an aluminum ore, a raw material for the production of alumina and alumina-containing refractories. The content of alumina in commercial bauxites ranges from 40% to 60% and more. It is also used as a flux in ferrous metallurgy.
But here is a paradox: with a huge number of minerals and rocks containing aluminum, bauxite deposits - the main raw material for industrial production aluminum are quite rare. In Russia, there are bauxite deposits in Siberia and the Urals. They are also of industrial importance. alunites and nephelines .
(Alunite, alum stone (fr. alunite - alum) - a mineral of composition K 2 SO 4 * Al 2 (SO 4) 3 * 4Al (OH) 3 or KAl 3 (SO 4) 2 (OH) 6. Color white, gray .
Nepheline (eleolite) is a rock-forming mineral, potassium and sodium aluminosilicate of orthosilicic acid (Na,K)AlSiO 4.)
Education precious stones
The corundum family, to which ruby and sapphire belong, has a very simple chemical formula- Al 2 O 3: the corundum molecule contains two aluminum atoms and three oxygen atoms.
Pure corundum is a colorless substance, but ideal gemstones rarely form in nature, and usually corundum is colored. Chromium and vanadium are the very accessory elements that give the ruby its characteristic red color; blue sapphire owes its color to iron and titanium, while green, yellow and pink sapphires owe their color to other combinations of satellite elements.
 The name "corundum" comes from the ancient names of this mineral: from the Tamil kurundam and kurund in Hindi.
The name "corundum" comes from the ancient names of this mineral: from the Tamil kurundam and kurund in Hindi.
The ancient Greeks mined corundum on the island of Naxos in the Aegean Sea, and today Naxos remains the main supplier of abrasive emery, used in industry in the form of powder, in everyday life we meet it in the form of nail files.
Pure corundum is colorless, today it is used as a decorative stone, in watchmaking, and as an abrasive.
There are deposits of corundum in many countries of the world.
 In everyday life, the word "sapphire" is associated exclusively with blue stones. The traditional colors of sapphires range from pale blue to deep blue (indigo). Sapphires of other colors are commonly referred to as "fantasy apphires" and include black, purple, green, taupe, yellow, orange, and white. Sapphires, as precious stones, were recognized as early as the 8th century BC. The rulers of ancient Persia believed that the sky was blue because sapphires were reflected in it. Different shades of sapphires are due to impurities of iron and titanium, and there are striped and spotted stones. The inclusions present in sapphires reflect light, resulting in an effect called "silk". The most transparent and colorless variety of sapphire is called leucosapphire.
In everyday life, the word "sapphire" is associated exclusively with blue stones. The traditional colors of sapphires range from pale blue to deep blue (indigo). Sapphires of other colors are commonly referred to as "fantasy apphires" and include black, purple, green, taupe, yellow, orange, and white. Sapphires, as precious stones, were recognized as early as the 8th century BC. The rulers of ancient Persia believed that the sky was blue because sapphires were reflected in it. Different shades of sapphires are due to impurities of iron and titanium, and there are striped and spotted stones. The inclusions present in sapphires reflect light, resulting in an effect called "silk". The most transparent and colorless variety of sapphire is called leucosapphire.
 Usually sapphires are found in the form of crystals having a tabular pyramidal or rhombohedral shape, as well as a barrel shape. Sapphires are characterized by repeated twinning. All sapphires are pleochroic: as soon as the stone is turned, its color changes.
Usually sapphires are found in the form of crystals having a tabular pyramidal or rhombohedral shape, as well as a barrel shape. Sapphires are characterized by repeated twinning. All sapphires are pleochroic: as soon as the stone is turned, its color changes.
Star-shaped stones are those in which several inclusions of rutile, similar to thin needles, reflect light in such a way that a twinkling six-pointed star appears. This effect is called asterism.
The most valuable sapphires are mined in Kashmir. These sapphires have a rich velvety luster.
Since 1902, they began to produce synthetic sapphires obtained from a melt of aluminum oxide with the addition of titanium.
Sapphire padparadsha
Padparadscha is an extremely rare variety of sapphire with a delicate pinkish-orange color due to the presence of small amounts of chromium, iron and vanadium. The name comes from the Sinhalese padmaragaya, which means "lotus flower".
The most expensive stone of all sapphires. It is mined in Sri Lanka.
 Rubies are extremely rare gemstones. Rubies are known different shades red color - from pinkish to brownish-red. The intensity of the red color depends on the amount of chromium that enhances the color. The brownish tint of the ruby indicates the presence of iron in them. The name comes from the Latin word ruber, which means red.
Rubies are extremely rare gemstones. Rubies are known different shades red color - from pinkish to brownish-red. The intensity of the red color depends on the amount of chromium that enhances the color. The brownish tint of the ruby indicates the presence of iron in them. The name comes from the Latin word ruber, which means red.
Rubies are mentioned in the Bible. In Sri Lanka, they have been mining for more than two and a half thousand years, and in Burma since the 6th century.
Rubies are found in crystalline limestone along with mica graphite, pyrrhotite, etc.
Ruby is a hard stone, but twin crystals break quite easily.
In 1902, the French chemist Auguste Verneuil developed a method for producing synthetic rubies from alumina and a dye.
Physical Properties
Aluminum oxide Al 2 O 3 is a white refractory powder, melting point 2044°C, boiling point 3530°C, density 4 g/cm3, hardness close to diamond. Several crystalline forms of aluminum oxide are known, up to 2044°C the crystalline modification of α-Al 2 O 3 - corundum is stable.
Its crystal structure is a two-layer densest spherical packing of oxygen ions, in the octahedral voids of which aluminum ions are located, the lattice is rhombohedral.
Chemical properties of Al 2 O 3
In air, aluminum is covered with the thinnest, but very dense oxide film, which protects the metal from further oxidation. In this regard, its surface usually does not have a shiny, but a matte appearance.
The oxide film formed on the surface of aluminum under atmospheric conditions is usually less than 1 nm thick, but is very strongly bonded to the metal. Films artificially obtained by the action of oxidizing agents are much thicker. A good protective film can be obtained, for example, by immersing aluminum in a solution containing 20% Na 2 SO 4 and 10% HNO 3 . With the help of selected fillers, such films can be given different colors.
On the contrary, after contact of aluminum with a solution of HgCl 2, this film becomes so loose that it no longer protects the metal from further oxidation. As a result, it quickly overgrows with a “beard” of aqueous oxide (Al 2 O 3 ·xH 2 O) and gradually oxidizes completely. The resulting aqueous oxide, both by itself and after dehydration by heating, has a high sorption activity.
When heated, the resistance of the oxide film is significantly reduced. Of particular note is the possibility of noticeable solubility of aluminum when it is boiled with dilute solutions of certain organic acids.
The ease of dissolution of aluminum in strong alkalis is due to the removal of a protective oxide film from it according to the scheme:
Al 2 O 3 + 2KOH - + 3 H 2 O = 2K.
Al 2 O 3 + 2 OH - + 3 H 2 O \u003d 2 Al (OH) 4 -
Since Al is much more to the left of hydrogen in the voltage series, the exposure of a clean metal surface is immediately accompanied by reactions according to the schemes:
2Al + 6H + (from water) \u003d 2Al + 3 + 3H 2 and 2Al + 3 + 8 OH - \u003d 2Al (OH) 4 -.
The balance of the first of them is constantly shifting to the right due to the second. The dissolution in alkalis of other active metals proceeds similarly, the hydroxides of which are amphoteric (Sn, Zn, etc.).
Aluminum oxide is a white, very refractory and water-insoluble mass. Natural Al2O3(mineral corundum), as well as artificially obtained and then strongly calcined, is distinguished by high hardness and insolubility in acids.
Aluminum oxide - amphoteric oxide with a predominance of basic properties; does not react with water.
1. Reacts with acids and alkali solutions:
a. As basic oxide:
Al 2 O 3 + 6HCl \u003d 2AlCl 3 + 3H 2 O
b. As an acid oxide:
Al 2 O 3 + 2NaOH + 3H 2 O \u003d 2Na
2) Alloys with alkalis or alkali metal carbonates:
Al 2 O 3 + Na 2 CO 3 = 2NaAlO 2 (sodium aluminate) + CO 2
Al 2 O 3 + 2NaOH \u003d 2NaAlO 2 + H 2 O
Al 2 O 3 + 2KOH \u003d 2KAlO 2 (metaluminate K) + H 2 O
Fusing Al 2 O 3 with alkalis, high molecular weight metaoxoaluminates are obtained.
In aluminosilicates, aluminum plays the same role as silicon: both of these elements form a mixed compound - aluminate-silicate.
Crystalline modifications of Al 2 O 3 are chemically very stable and do not interact with water and acids. Aluminum oxide (sesquioxide) can be converted into a soluble state by fusion with alkalis or K 2 S 2 O 7 according to the reactions:
Al 2 O 3 + 2 NaOH \u003d H 2 O + 2 NaAlO 2
Al 2 O 3 + 3 K 2 S 2 O 7 \u003d Al 2 (SO 4) 3 + 3 K 2 SO 4.
Application of Al 2 O 3
1. Aluminum oxide - raw material for aluminum production; produced from aluminum-containing ores, preim. bauxites. Aluminum is also obtained from nephelines, kaolin, alunites by the aluminate or chloride method. Raw material in aluminum production, catalyst, adsorbent, refractory and abrasive material.
The first attempts to obtain aluminum were made only in mid-nineteenth century. An attempt made by the Danish scientist H.K. Oersted was successful. To obtain it, he used amalgamated potassium as a reducing agent for aluminum oxide. But what kind of metal was obtained then it was not possible to find out. Some time later, aluminum was obtained by the German chemist Wöhler, who obtained aluminum using the heating of anhydrous aluminum chloride with potassium metal.
Many years of work of the German scientist were not in vain. For 20 years, he managed to prepare granular metal. It turned out to be similar to silver, but was much lighter than it. Aluminum was a very expensive metal, and until the beginning of the 20th century, its value was higher than that of gold. Therefore, for many, many years, aluminum has been used as a museum exhibit.
Around 1807, Davy tried to carry out the electrolysis of alumina, received a metal that was called aluminum (Alumium) or aluminum (Aluminum), which is translated from Latin as alum.
Obtaining aluminum from clays was of interest not only to chemical scientists, but also to industrialists. It was very difficult to separate aluminum from other substances; this contributed to the fact that it was more expensive than gold. In 1886, the chemist Ch.M. Hall proposed a method that made it possible to obtain metal in large quantities. Conducting research, he dissolved aluminum oxide in the AlF 3 nNaF cryolite melt. The resulting mixture was placed in a granite vessel and passed through the melt constant electricity. He was very surprised when, after some time, he found plaques at the bottom of the vessel. pure aluminum. This method is still the main one for the production of aluminum on an industrial scale. The resulting metal was good for everything, except for the strength, which was necessary for the industry. And this problem has been solved. German chemist Alfred Wilm fused aluminum with other metals: copper, manganese and magnesium. The result was an alloy that was much stronger than aluminium. On an industrial scale, such an alloy was obtained in the German town of Düren. This happened in 1911. This alloy was named duralumin, after the town.
In industry, aluminum is obtained by electrolysis of a solution of alumina Al 2 O 3 in molten cryolite Na 3 AlF 6 . The process is carried out at temperatures of about 1000 ° C in special electric furnaces. The electrolysis of Al 2 O 3 can be represented by the following conditional scheme. In solution, the oxide dissociates into ions
Al 2 O 3 ↔Al 3+ + AlO 3- 3
Al 3+ ions are discharged at the cathode: Al 3+ +3e - \u003d Al 0
The process occurs at the anode: 4AlO 3- 3 - 12e - \u003d 2Al 2 O 3 + 3O 2
Oxygen is released at the anode, and liquid aluminum is released at the cathode. The latter is collected at the bottom of the furnace, from where it is periodically released. The cathode is the body of the electrolyser, on which liquid aluminum is released. Oxygen is released on the graphite anode, which oxidizes graphite to carbon oxides. As the anode burns out, it is increased. Because liquid aluminum has a higher density than melt, it collects at the bottom of the electrolytic cell.
Purification of aluminum from impurities is difficult, so it is necessary that the raw materials themselves be pure to obtain it. Cryolite is usually prepared artificially by jointly dissolving Al (OH) 3 and soda in hydrofluoric acid according to the reaction:
3 Na 2 CO 3 + 2 Al (OH) 3 + 12 HF \u003d 2 Na 3 AlF 6 + 3 CO 2 + 9 H 2 O.
Natural bauxites, which include 50-60% Al 2 O 3 and a number of impurities (SiO 2 , Fe 2 O 3 , etc.), are subjected to preliminary chemical processing in order to isolate from them sufficiently pure aluminum sesquioxide (containing no more than 0.2 % SiO 2 and 0.04% Fe 2 O 3). The methods of such processing are highly dependent on the composition of the original bauxite and are quite complex.
The aluminum smelting furnace consists of an iron box, the inner walls and bottom of which are lined with a heat-insulating layer of refractory materials and, on top of it, a thick carbon lining, which serves as a cathode during electrolysis. A massive carbon electrode is used as an anode. The process is carried out at a temperature of about 960 ° C, a voltage of about 5 V and a current strength of about 140 thousand A. The released oxygen forms CO and CO 2 with the anode coal. At the same time, small amounts of CF 4 are obtained due to the slight release of fluorine. Due to the combustion of the anode, it has to be gradually lowered down. The side walls of the oven (and most of surfaces of the liquid) are covered with a solid crust of electrolyte, which prevents their separation by the gases released at the anode and protects the melt from cooling. During the operation of the furnace, Al 2 O 3 (and some cryolite) is periodically added to it, and the molten metal is removed.
Aluminum smelting is very energy intensive: a ton of metal requires about 10 thousand kWh of electricity. Its primary cleaning is carried out by blowing chlorine. Sales metal usually contains 99.7% aluminum. Along with other impurities (mainly Si and Fe), it also contains traces of gallium.
The constant and ever-increasing demand for aluminum in the 1980s could no longer satisfy bauxite reserves. According to scientists, by the middle XXI century the bauxite source will begin to dry up. There is an urgent need to find other types of raw materials. For the first time in world practice, faced with the same problem, it was in the USSR that alumina (aluminum oxide - Al 2 O 3) began to be obtained from alunite - white or grayish-yellow alum (potassium and aluminum hydrogen sulfates containing up to 37% Al 2 O 3) .
2. The high bond strength of Al-O-Al and the dense crystal structure predetermine the high melting point (about 2050°C), hardness and refractoriness of aluminum oxide. So, corundum is second only to diamond in hardness and is used as an abrasive material in the form of corundum circles and emery. As a refractory material, it is also widely used artificially, obtained from bauxite, strongly calcined Al 2 O 3, called alundum. Due to its high hardness, corundum monocrystals (in particular, rubies) are artificially obtained as reference stones in precision mechanisms. Artificial rubies are used as quantum generators (lasers).
Usually, natural corundum contaminated with iron oxide, due to its extreme hardness, is used for the manufacture of grinding wheels, bars, etc. In finely crushed form, it is called emery for cleaning metal surfaces and making sandpaper. For the same purposes, aluminum oxide is often used, obtained by fusing bauxite (technical name - alund).
Pure aluminum oxide (mp 2050, bp 3500°C) is directly used in the production of dental cements. So, the powder of one of the types of high-quality dental cement is obtained by fusion at 700-800 ° C and subsequent grinding of a carefully prepared mixture of the following composition: 28.4% Al 2 O 3, 20.9-SiO 2, 19.7-Na 2 SiF 6 , 19.0-CaSiF 6 , 3.9-CaCO 3 , 4.1-H 3 PO 4 , 4.0-H 3 AsO 4 . The liquid for mixing such cement is a strong solution of Al(H 2 PO 4) 3 .
Aluminum oxide products have very high mechanical strength and retain it up to 1800 °C. Their chemical resistance is also exceptionally high. However, they conduct heat well and tolerate temperature fluctuations. By sputtering molten alumina, an effective protective coating can be created on metals.
Fusion of equal mass amounts of Al 2 O 3 and SiO 2 with subsequent blowing of their melt was obtained glass fiber (“ fiberfrax”), characterized by high thermal stability and high chemical resistance. It does not change its properties up to 1250°C, melts only above 1600°C and is especially suitable for the manufacture of heat-insulating materials.
On the basis of corundum, a heavy-duty artificial stone was designed - “ microlite". It consists of very fine (on the order of microns) grains of corundum with a small addition of glassy binding material. Microlithic cutters retain their extreme hardness up to 1200 °C and therefore allow very high metalworking speeds.
The idea of an optical quantum generator was realized for the first time (1960) on a ruby crystal (“ laser”) - a device that creates a directed beam of monochromatic (i.e., having one specific wavelength) radiation in the visible region of the spectrum or near it. The action of a laser (as well as its related “maser”, which generates a similar beam of short radio waves) is based on the release of energy due to a certain decrease in the energy level of many identical particles that occurs simultaneously.
Conclusion
The scope of aluminum oxide is very wide, fascinating story its discovery dates back to ancient times. Also in ancient rome people sought to learn about this substance, learning more and more about its properties. And already now there are new nano-technologies in which aluminum oxide plays leading role. Perhaps in the future with the help of this substance, will be developed new technology, one more, and maybe several types of precious stones will appear, obtained in the same way as the existing ones, artificially.
Information sources
1. Encyclopedia. Geology. M., "Avanta +" 1995, pp. 304,306,357.
2. Akhmetov N.S. General and inorganic chemistry. M., "Higher School" 1998. pp. 430-432.
3. Oldershaw.K. Atlas of gems.
4. Komkova E.G. A group of chemical asteroids. M., "Enlightenment" 1984. p. 404,405
5. Website: http://schoolchemistry.by.ru Aluminum oxide.
6. Website: http://www.alhimikov.net Aluminum.
Author Chemical Encyclopedia b.b. I.L.KnunyantsALUMINUM OXIDE(alumina) Al 2 O 3 , colorless crystals; melting point 2044°C; boiling point 3530 °C. The only stable up to 2044 ° C crystalline. modification of ALUMINUM OXIDE o.-Al 2 O 3 (corundum): rhombohedral lattice, a \u003d 0.512 nm, \u003d 55.25 ° (for hexagonal installation a \u003d 0.475 nm, c \u003d 1.299 nm, space group D 6 3d, z = 2); density 3.99 g / cm 3; N ° pl 111.4 kJ / mol; temperature dependence equations: heat capacity C ° p \u003d \u003d 114.4 + 12.9 * 10 -3 T - 34.3 * 10 5 T 2 JDmol * K) (298T 1800 K), vapor pressure Igp (Pa) \u003d -54800 /7 + 1.68 (up to ~ 3500 K); temperature coefficient of linear expansion (7.2-8.6) * 10 -6 K -1 (300T1200 K); thermal conductivity of the sample sintered at 730°C 0.35 W/(mol*K); Mohs hardness 9; the refractive index for an ordinary beam is n 0 1.765, for an extraordinary beam it is 1.759. See table.
THERMODYNAMIC PROPERTIES OF ALUMINUM OXIDES 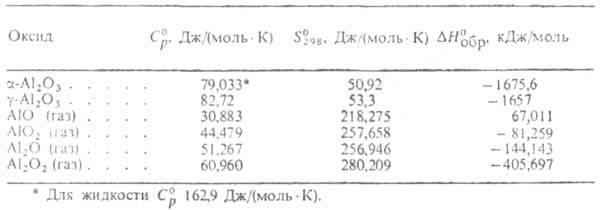
Modification-Al 2 O 3 occurs in nature in the form of the mineral corundum, which often contains oxides of other metals in dissolved form, giving it a different color. Transparent colored crystals-precious stones (sapphires, rubies, etc.). Corundum can be obtained artificially as a result of thermodynamic decomposition of rhombic. modification of AlOOH-diaspore or polymorphic transitions of metastable forms of Al 2 O 3 (etc.), which are formed during the decomposition of crystalline. modifications of Al (OH) 3 -gibbsite and bayerite and AlOOH-boehmite (see Aluminum hydroxide). These processes can be represented by the following diagram: 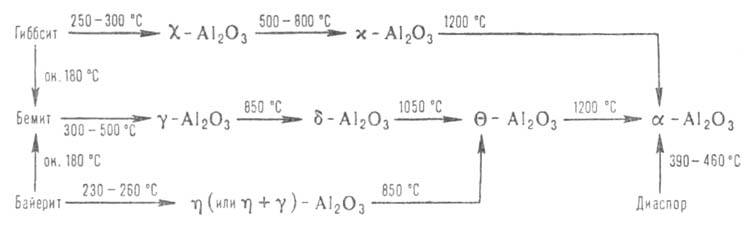
Modification Al 2 O 3 has a tetragon, crystalline. a spinel-type lattice (a = 0.562 nm, c = 0.780 nm); density 3.3-3.4 g/cm 3 ; contains structurally bound water in the amount of 1-2%. There is also amorphous ALUMINUM OXIDO. - alumogel formed during dehydration of gel-like Al(OH) 3 and which is a porous, sometimes transparent substance. ALUMINUM OXIDE about. insoluble in water, freely soluble in molten cryolite. Amphoterene. With NH 3 -H 2 O does not react. Chemical activity synthetic. A. o. greatly decreases with increasing temperature. Natural and artificial (formed above 1200 ° C) corundum in air under normal conditions are chemically inert and non-hygroscopic. OK. 1000°C intense interaction with alkalis and alkali metal carbonates, giving aluminates. Slowly reacts with SiO 2 and acidic slags to form aluminosilicates. During fusion, interaction with KHSO 4 . Corundum, formed from diaspore at 500-600 ° C, also interacts with solutions of acids and alkalis. Alumogel and Al 2 O 3 obtained by firing Al hydroxides at ~550°C are very hygroscopic and chemically active, react with solutions of acids and alkalis.
Raw materials for obtaining ALUMINUM OXIDO. - bauxites, nephelines, alunites, etc. (see Aluminum ^ With a ratio in ores of Al 2 O 3: SiO 2 > 6-7, they are processed according to the Bayer method (main method), with Al 2 O 3: SiO 2< 6 (высококремнистое сырье) - спеканием с известью и содой.
According to the Bayer method, bauxite crushed in ball mills is leached in autoclaves with a recycled alkaline solution of Na aluminate (after part of Al 2 O 3 is separated from it) at 225-250°C. In this case, aluminum passes into solution in the form of Na aluminate. In the case of bauxite containing gibbsite, leaching can be carried out at 105°C and normal pressure in apparatus with a stirrer. Aluminate solutions are diluted with water, the sludge is separated and subjected to decomposition in apparatuses with a stirrer or airlift for 30-70 hours, and approx. 1/2 of the resulting Al(OH) 3 . It is filtered off and calcined in rotary kilns or in a fluidized bed at ~ 1200°C. The result is alumina containing 15-60% Al 2 O 3 . The mother liquor is evaporated and fed to the leaching of a new batch of bauxite.
According to the second method, high-silicon crushed ore (nepheline, etc.) is mixed with soda and limestone and sintered in rotary kilns at 1250-1300: C. The resulting mass is leached with an aqueous alkaline solution, the Na aluminate solution is separated from the sludge, then freed from SiO 2, precipitating it in an autoclave at a pressure of approx. 0.6 MPa, and then with lime at atmospheric pressure, and decompose the aluminate with gaseous CO 2 . The resulting Al(OH) 3 is separated from the solution and calcined at a temperature of approx. 1200°C. When processing nepheline, in addition to alumina, Na 2 CO 3 , K 2 CO 3 and cement are obtained. In the production of alumina from alunites, H 2 SO 4 and K 2 SO 4 are simultaneously obtained. Alunite ore is fired at 500-580°C to restore. atmosphere and treated with NaOH solution according to the Bayer method. Single crystals are grown by zone melting, according to the Verneuil or Czochralski method.
Synthetic Al 2 O 3 - an intermediate product in the production of Al (the main area of use), a refractory and abrasive material. It is also used in the production of ceramics. cutters, electrical engineering. ceramics. Monocrystals - laser material, reference stones of watch mechanisms, jewelry stones. Natural corundum-abrasive (corundum circles, emery) and refractory material. Alumogel, Al 2 O 3 and its mixture with -Al 2 O 3 - adsorbents for drying gases (for example, H 2 , Ag, C 2 H 2) and liquids (aromatic hydrocarbons, kerosene, etc.), in chromatography; catalysts (eg, dehydration of alcohols, isomerization of olefins, decomposition of H 2 S); carriers for catalysts (for example, Co-MoO 3 , Pd, Pt).
World production of ALUMINUM OXIDE about. OK. 30 million tons/year (1980). Other A. o. are also known. (see table) existing in the gas phase.
Chemical encyclopedia. Volume 1 >>
Aluminum oxide insulation belongs to heat resistance class C. Since the melting point of aluminum oxide is very high, about 2050 C, it is possible to heat oxidized aluminum wire to the metal melting point (see page. However, the disadvantages of oxide anodized insulation are its low flexibility and noticeable due to film porosity hygroscopicity In those cases where particularly high heat resistance is not required, oxide insulation can be impregnated and varnished.
The physical properties of oxides change regularly and, accordingly, the change in the properties of elements by periods and groups. On fig. 80 shows the dependence of the melting point of oxides on the element's serial number. At ordinary temperatures, metal oxides are solid crystalline substances, non-metal oxides can be in gaseous (SOa, CO, etc.), liquid (H2O, etc.) and solid (PaOz, P2O5, SiO2, etc.) states of aggregation.
Similarly, vanadium and molybdenum catalysts cannot be easily reduced by ethylene at its polymerization temperature, so a promoter serving as a reducing agent must be used to achieve high activity. As shown in Table. 6, the melting point of the oxide rises sharply on going from chromium to vanadium and molybdenum. The low melting point of CrO3 ensures its mobility over the surface of silicon oxide and, thus, high dispersion.
The above conditions determine the requirements for the metal processed by oxygen cutting. First of all, the melting point of the metal must be higher than the melting point of the oxides.
The behavior of the oxide makes it possible, to a certain extent, to predict the reaction of one or another cation during calcination on coal. For example, manganese compounds will not sublimate, since the melting point of manganese oxide is 1650 C. Thallium and germanium sublimes can be obtained, since thallium oxide melts at 300 C, giving a black sublimation, and germanium oxide is able to sublimate from a solid state at 700 C. These metals are not included in the above table as they are rarely encountered in practice. qualitative analysis. The formation of a bead is associated with the ability of metal oxide to be reduced by carbon. For example, gallium will not give a beetle, since its oxides are not reduced by coal.
After general purification, the roasting gas obtained from pyrite must be subjected to special purification to remove dust and fog residues and, mainly, arsenic and selenium compounds, which are then disposed of. Special gas purification includes operations of cooling it to a temperature below the melting points of arsenic oxide (315 C) and selenium (340 C) in towers irrigated successively with 50% and 20% sulfuric acid, removing sulfuric acid mist in wet electrostatic precipitators and final gas dehydration in scrubbers irrigated with 95% sulfuric acid. The roasting gas exits the special purification system at a temperature of 140 - 50 C.
In the last three cases of this table (Al, Mg and Zn), the temperature of the anode and cathode is determined by the melting temperature of the oxides with which the corresponding metals are covered during arcing in air.
For the normal flow of the oxyfuel cutting process, the following conditions must be met. The ignition temperature of the metal must be below the melting point. The melting point of metal oxides must be lower than the melting point of the metal itself. The oxides must be fluid. The heat released during the combustion of the metal must be large enough to maintain a continuous process. These conditions are fully met by low-carbon steels. Low alloy structural steels cut satisfactorily. High-alloy chromium and chromium-nickel steels, cast iron, and non-ferrous metals are not amenable to conventional oxy-fuel cutting. For gas cutting of these metals, fluxes are used that dissolve relatively refractory oxides and increase heat generation during cutting.
The protective properties of chloride films are low; in some cases, metals ignite in a stream of chlorine. In table. 6.4 shows the melting points of chloride salts of a number of metals. For comparison, the melting points of oxides and sulfides are given.
If we consider the properties of metals in a state with an oxidation state of 5, the following should be noted: oxides are dense, stable, inert substances. In terms of the size of the atom and ion, niobium and tantalum are close to each other. This is also reflected in the properties of oxides, the formation temperature of which is high for niobium and tantalum, as well as the melting point of oxides, and higher oxides NbsOs and Ta2O5 are practically insoluble in water. If we compare ions of the same oxidation state, we can notice an increase in metallic properties. The acidic properties of hydroxides are higher than in the titanium subgroup, and fall from vanadium to tantalum. Vanadium hydroxide is a weak acid, while niobium and tantalum hydroxide are amphoteric compounds. Since these elements do not have d-orbitals filled, it means that they are able to form complex compounds. The niobium atom in NbF5 has a positive charge, since fluorine pulls electrons away from Nb.
Welding of aluminum and its alloys is difficult due to its special thermophysical properties. Upon contact with air, a dense thin film of Al2O3 oxide is formed on the aluminum surface, which protects the metal from further corrosion, but at the same time worsens the welding conditions, since the melting point of aluminum oxide is 2050 C, therefore, oxide must be removed from the aluminum surface before welding. Aluminum is easily oxidized during welding, and the oxide film formed on the drops and in the bath contaminates the seam. In the molten state, aluminum dissolves hydrogen well, which, at an increased cooling rate caused by the high thermal conductivity of the metal, does not have time to be released at the moment of crystallization and causes porosity.
(lat. Aluminum) - chemical element Group III periodic system Mendeleev, atomic number 13, atomic mass 26,981.
The earth's crust contains 8.80% aluminum; it is the third most abundant element on our planet after oxygen and silicon. It is a part of clays, feldspars, micas. The most important aluminum mineral - bauxite contains 28-60% alumina - aluminum oxide Al2O3.
- the most common metal in nature, but in pure form it was first obtained by the Danish physicist X. Oersted only in 1825, later than many metals. Aluminum is a light, silvery metal that conducts heat and electricity well. Aluminum is reactive. Easily oxidized by oxygen, however, only on the surface. The A12O3 oxide film reliably protects the metal from further oxidation. But if aluminum powder or aluminum foil strongly heated, then the metal burns with a dazzling flame, turning into the same oxide. Aluminum dissolves in hydrochloric and sulfuric acids, as well as in aqueous solutions alkalis. It actively reacts with halogens. Aluminum hydroxide A1 (OH) 3 - a typical amphoteric compound - a white, translucent, gelatinous substance.
In most compounds, aluminum is trivalent, but at high temperatures it is also capable of exhibiting an oxidation state of + 1. Of the compounds of this metal, the oxide A12O, is obviously the most important. This formula expresses the composition of both alumina and the very hard mineral corundum. Corundum crystals colored by impurities in Blue colour, called sapphire, in red - ruby. Rubies and sapphires are now produced artificially on an industrial scale.

Long after aluminum was obtained in its pure form, this metal remained very rare and expensive. Select it from natural compounds difficult, since it is very tightly bound in them with oxygen and other elements. Aluminum can be obtained by electrolysis of the melt aluminum oxide, but this substance melts only at 2050°C. therefore, too much energy is required.
Aluminum could become a technically important metal only if a way was found to lower the melting point of aluminum oxide to at least 1000°C. Such a "detour" was found almost simultaneously by the American C. Hall and the Frenchman P. Eru. They found that alumina dissolves well in molten cryolite, a mineral of composition P3 3KaR. This melt is subjected to electrolysis at a temperature of ~ 950°C for aluminum production. True, soon after the discovery of Hall and Eru it became clear that the reserves of cryolite on Earth are very limited. The production of synthetic cryolite was organized, and now aluminum is the cheapest of all non-ferrous metals.
Most of the aluminum produced in the world is used to produce light alloys; copper, magnesium, silicon, and zinc are added to aluminum. manganese to increase its strength. Aluminum is called the main metal of aviation technology today, it is also needed in transport engineering, in the chemical industry and electrical engineering. Aluminum is a very durable metal: it can be processed by pressure at normal and slightly elevated temperatures, products from it can be prepared by rolling, drawing, stamping, forging, pressing.